Our medical device experience is defined by our unique blend of advanced engineering, low-volume production expertise, and dedicated support for established medical device companies. Our team can integrate seamlessly with your quality system, offering robust technology and concept development that fast-tracks the path from prototype to production.
We’re currently pursuing our ISO 13485 certification for our production space. Reach out if you have any questions!
We engineer diagnostic and therapeutic tools for rapid, on-site use.
We design and develop a range of custom surgical tools and instruments to be used during complex procedures.
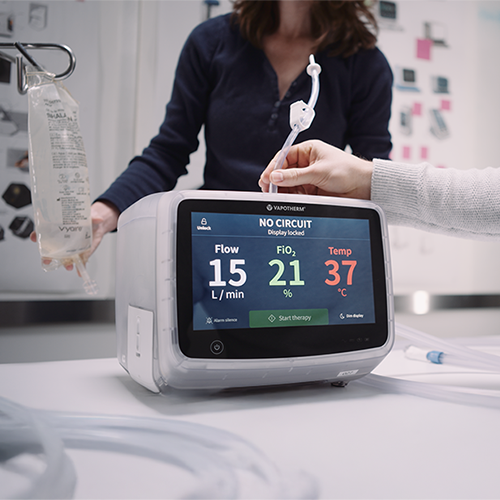
We engineer respiratory support devices, including ventilators, oxygen concentrators, monitors, and patient-assistance tools.
We develop medical devices for at-home sample collection and preparation.
We use rapid prototyping and design for manufacturing to create implantable devices.
We design and develop devices for diagnosing a wide range of medical conditions.
Medical Device Expertise
We thrive on the “fuzzy front end” of medical device innovation, where our team embraces open-ended challenges to test the feasibility of new ideas. Our passion is problem-solving, and we excel at assessing new technologies and prototyping to determine if a concept can be made a reality, providing a rapid and flexible approach to early-stage development.


We have a certified BSL-2A Biosafety Lab and ISO Class 7 Clean Rooms right here at Re:Build Fikst. While our low-volume production projects will be covered by this certification, our engineering services typically operate under the client’s existing quality system. This approach ensures documentation and risk management remain client-led, allowing our team to stay focused on what we do best: implementing ideas and building devices.
Vapotherm partnered with Re:Build Fikst to develop the HVT 2.0, a next-generation respiratory therapy device. The goal was to create a highly reliable, precise device to treat complex respiratory conditions and reduce the need for invasive procedures like endotracheal intubation. The project also included the development of a disposable patient circuit for clinical use.
Fill out the form below and someone from our team will be in touch with you shortly.